Treating DIPG/DMG
Radiation
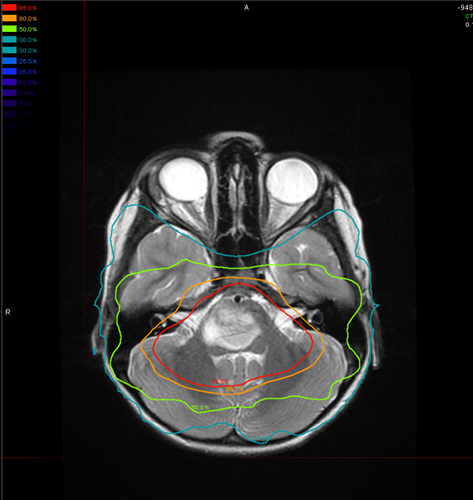
While essentially palliative, focal radiotherapy remains the primary approach to treating DIPG and results in symptom improvement or at least stabilization for most children. Standard treatment generally consists of 1.8-Gy fractions delivered once daily, 5 days a week, for a cumulative target dose of 54 Gy. Hyperfractionated doses up to 72 Gy have not shown improved efficacy in pediatric patients, and hypofractionated radiotherapy may lead to slightly inferior outcomes.1 In some brain tumors, protons are superior to photon radiation because they can spare nearby normal brain tissue from potentially harmful effects of radiation. However, they have not been shown to have an advantage in the treatment of DIPG. More patients demonstrate a clinical response to radiotherapy (85%) than an evaluable radiological response (50%), but little or no correlation has been shown between radiological response and either clinical response or survival.2 The benefits of radiotherapy last an average of 6 months.
So far, clinical studies of radiosensitizing agents such as cis-platinum,tipifarnib and gadolinium texaphyrin have not shown any benefit versus radiation alone.
Clinical Trials
Clinical studies of chemotherapy – given during and after radiation – have examined multiple conventional agents including high dose chemotherapy/autologous stem cell rescue as well as multiple targeted agents (e.g. VEGF-inhibitor bevacizumab, EGFR-inhibitor erlotinib or gefitinib, tyrosine kinase inhibitor imatinib) without showing improvement in survival. Frappaz et al. have published data suggesting some improvement in median overall survival with pre-radiation high-dose methotrexate, BCNU, cisplatin, and tamoxifem, but long-term overall survival was still poor, just 4% at 3 years.3 Massimino et al. reported on three protocols incorporating neo-adjuvant chemotherapy: the median overall survival was 9-13 months, but a study using vinorelbine reported two long-term survivors, alive at 31 and 48 months after diagnosis.4 A Children’s Oncology Group study of temozolomide concluded that there is little justification for using this agent alone or in combination with other agents in DIPG.5 A recent Phase I study of the VEGFR2- and EGF-inhibitor vandetanib from St. Jude demonstrated poor outcomes overall but two-year progression-free survival in a very small number of patients.6
The benefit of chemotherapy at relapse remains uncertain. The feasibility of palliative reirradiation with chemotherapy has been reported to improve symptoms and delay further progression with minimal toxicity. Six patients at MD Anderson received reirradiation for progressive DIPG defined as progression after initial chemoradiation and salvage chemotherapy. The interval between the initial radiation therapy and reirradiation was 8 to 28 months. Reirradiation therapy was to a dose of 20 Gy (n=4) or 18 Gy (n=1). Patients who were most likely to benefit may be those with prolonged response to initial therapy and a long interval since initial radiation.
Post-radiation chemotherapy has shown no benefit. The benefit of chemotherapy at relapse remains uncertain. Chemotherapy for pediatric DIPG patients is best approached in the context of a clinical study. To date, the treatment approach for DIPG patients is still very heterogeneous and only a minority of patients are included in clinical trials. Given the rarity of DIPG, there is a shift towards (inter-)national trials to facilitate the identification of potentially effective therapeutics in the future.
- Janssens, G.O., et al., The role of hypofractionation radiotherapy for diffuse intrinsic brainstem glioma in children: A pilot study. International journal of radiation oncology, biology, physics, 2009. 73(3): p. 722-6.
- Hargrave, D., U. Bartels, and E. Bouffet, Diffuse brainstem glioma in children: Critical review of clinical trials. The lancet oncology, 2006. 7(3): p. 241-8.
- Frappaz, D., et al., Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: Final results of bsg 98 prospective trial. Neuro-oncology, 2008. 10(4): p. 599-607.
- Massimino, M., et al., Diffuse pontine gliomas in children: Changing strategies, changing results? A mono-institutional 20-year experience. Journal of neuro-oncology, 2008. 87(3): p. 355-61.
- Cohen, K.J., et al., Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the children's oncology group. Neuro-oncology, 2011. 13(4): p. 410-6.
- Broniscer, A., et al., Phase i study of vandetanib during and after radiotherapy in children with diffuse intrinsic pontine glioma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 2010. 28(31): p. 4762-8.
- Veldhuijzen van Zanten SE, Jansen MH, Sanchez Aliaga E, et al. A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Expert Rev Anticancer Ther. 2014 Nov 29:1-8.
